British Medical Journal, Volume 295, Issue 6609, Pages 1306-1309. Saturday, 21 November 1987.
British Medical Journal, Volume 295, Issue 6609, Pages 1306-1309. Saturday, 21 November 1987.
A retrospective study was carried out to determine whether penile cancer, like cervical cancer, was associated with smoking and sexual behaviour. Altogether 244 men with penile cancer and 232 matched controls completed a questionnaire by post or telephone. Data on marital state, socioeconomic group, occupation, history of phimosis and balanitis, sexual behavior, and smoking were obtained. The results of statistical analyses confirmed that phimosis and balanitis were risk factors for penile cancer, but there was no epidemiological evidence for it being a sexually transmitted disease. Smoking was a risk factor with a dose response relation and remained associated with penile cancer even after adjustment for confounding factors.
Penile cancer is associated with smoking independently of phimosis; treatment of phimosis alone does not remove the risk caused by smoking.
Cancer of the penis is a serious health problem in many parts of the world. In the Western World the incidence of penile cancer varies from 0.5 to 1.5 per 100 000 men. In many parts of the world, however, it is the most common male cancer with an incidence of 2 to 5 per 100 000, constituting--for example, 12% to 22% of all male cancers in China, Uganda, and Puerto Rico.1 The incidence is extremely low in Israel and Moslem countries, where neonatal circumcision or circumcision during childhood is practised: only nine cases of penile cancer in Jewish men circumcised neonatally have been reported world wide,2 but a higher incidence has been reported in Moslems, who practice circumcision at 5-10 years of age.3
Circumcision has been known to be a negative risk factor since the beginning of this century.4 Known risk factors are phimosis and balanitis: phimosis has been reported in 50 to 98% of patients with penile cancer, and chronic suppuration and a history of balanitis are also very common.4-8
Smoking in women has been accepted as a genuine risk factor for cervical neoplasia.9,10 Winkelstein hypothesized that smoking causes squamoepithelial cancer not only in parts of the body in contact with smoke (lung, larynx) but also far from where it makes contact (urinary bladder, cervix) but by means of the circulatory system.11 This hypothesis may apply to penile cancer, which in 98% of cases is squamoepithelial.
Human papillomavirus has recently been implicated as a causal agent in penile cancer,12-16 which suggests that this cancer may be a sexually transmitted disease,17 with its female counterpart being cervical cancer.18-20 If penile cancer were sexually transmitted this would be reflected epidemiologically by measuring sexual behavior (low age at first intercourse and many sexualpartners), as for cervical cancer.
In Sweden an average of 50 men a years are diagnosed as having cancer of the penis, giving an incidence of 1.4 per 100 000.21 All cases of cancer are registered at the National Cancer Registry, which in 1958-80 registered 1064 new cases. As the median age of onset in Sweden is 65 to 69 years21 a large proportion of these patients were dead when we started this study. The population studied was all living men under 80 with cancer of the penis, who numbered 299. According to the regulations of the National Cancer Registry, the doctor who diagnosed or treated each patient must give her or her written permission to allow an interview with the patient. We traced the doctors for 247 patients, and they all gave their permission. Three patients were excluded from the study because of serious brain damage due to cerebrovascular disease.
For each patient we selected a control subject from the Swedish population registry. The controls were matched for age and geographical area. Fifteen of the 247 controls were excluded because they had brain damage due to cerebrovascular disease, had recently died, or had moved abroad, thusgiving a control group of 232 men.
All 476 men in both groups, most of whom were aged 60-80 years, were sent a questionnaire. This included questions about marriage, occupation, smoking habits, use of snuff, history of phimosis and balanitis, age at first intercourse, and the number of sexual partners during their life. The men were particularly asked not to answer questions on subects about which they were not absolutely sure to avoid biasing the results. The questionnaire was coded, and only we had access to the code, which guaranteed total anonymity and meant that no identification number would be registered when the results were statistically analysed. In cases in which there was no reply to the questionnaire, a maximum of two reminders were sent. On the fourth occasion patients were sent a letter asking permission for an interview by telephone; those who agreed were subsequently interviewed by telephone by one of us.
All information was entered into a computer. Initial significance tests were done with χ² or Student's τ tests as appropriate. To assess the simultaneous effect of all likely explanatory variables we analyzed multiway frequency tables. As patients were matched with controls in the design of the study we performed analyses with a logistic approach for matched data.22 Unmatched log linear analyses were also used, whose results were similar to those in the logisticanalyses.
The questionnaire was answered by 381 (80%) men, and another 43 agreed to be interviewed by telephone, giving a total response of 424 out of 476 (89%). A slightly higher proportion of patients (91%, 223) than controls (87%, 201) agreed to be interviewed. Not all subjects were able to answer all the questions. The most difficult to answer were those concerning sexual history. Of the 424 who responded, 22 (5%) did not know the number of sexual partners and 34 (8%) had forgotten how old they were when first had sexual intercourse. Those who could not answer either or both of these questions were equally distributed among patients and controls.
Penile cancer was not associated with a particular occupation nor was there any significant difference in its distribution among different socioeconomic groups.23 The incidence of penile cancer tended to be higher among lower classes; a comparison of classes 1 and 2 (lower) with classes 3-5 (higher) gave a χ² value of 3.73 (p=0.05). People in classes 1 and 2 also tended to smoke more often so that when socioeconomic group was controlled for smoking the significance of socioeconomic group vanished.
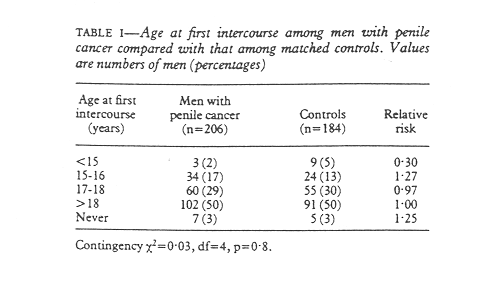
An equal proportion (88%) of men in the two groups were or had been married (197 patients, 175 for controls). There was no difference in age at first intercourse, with a median age of 18 for both groups and an equal distribution in all age groups (table I). As men with phimosis might have had a different sexual history from other men age at first intercourse was controlled for phimosis. This did not change the findings.
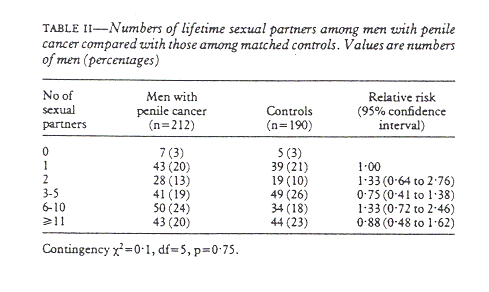
Only seven men with penile cancer and five controls said that they had never had intercourse with a woman (table II). On average, both groups of men had had intercourse with six to 10 women during their lives. There was no statistical difference between patients and controls, and this remained so when the number of sexual partners was controlled for phimosis.
As would be expected, there was a much greater prevalance of phimosis among men with cancer of the penis. (table III). The relative risk of having penile cancer among men with phimosis was 64.6, which was a highly significant excess risk. The large numbers of men with a history of one or more episodes of balanitis was also significant among those with penile cancer (table IV); the relative risk was 9.49 for men who had experienced one or more episodes of balanitis. As balanitis may be difficult to remember (the beginnings of penile cancer could be misinterpreted as balanitis by patients) those who stated that they had suffered from balanitis more than once were analysed separately, but balanitis remained a strong risk factor for penile cancer. Balanitis could also be secondary to phimosis and therefore phimosis was controlled for in one analysis. The relative risk with balanitis then decreased to 5.22 but was still significant (p < 0.001).
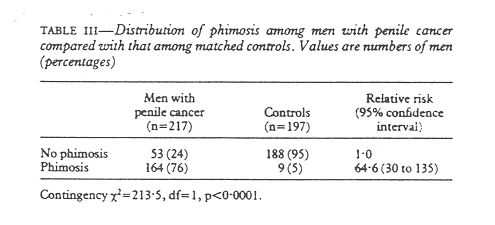
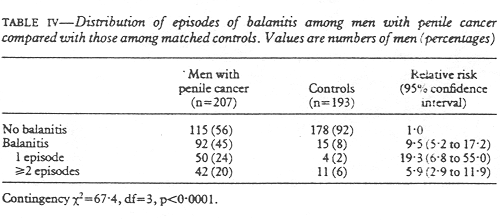
Table V shows the distribution of smoking in the two groups. Smoking had a significant effect on the prevalence of penile cancer even when the amount of smoking was not considered. When smokers were grouped according to the number of cigarettes smoked a day we saw a clear dose-response relation, with smokers of more than 10 cigararettes a day having a significantly higher risk than light smokers (1 to 10 cigarettes a day) (χ²=5.43, p=0.02). The relative risk of having penile cancer for smokers of more than 10 cigarettes a day was 1.88 (95% confidence level 1.10 to 3.19) when compared with light smokers and 2.22 when compared with non-smokers.
Table V shows that ex-smokers had a higher risk of having penile cancer than light smokers. This might have been because ex-smokers were not grouped according to the number of cigarettes that they had consumed a day. Had this been done ex-smokers would have been expected to have a relative risk closer to that of non-smokers. Thus we found a clear dose-response relation between smoking and penile cancer, but no effect of duration of smoking. This might have been because most men in this study had smoked for a very long time (88% for more than five years). We also asked about taking snuff but found no difference in the proportions using it(6%).
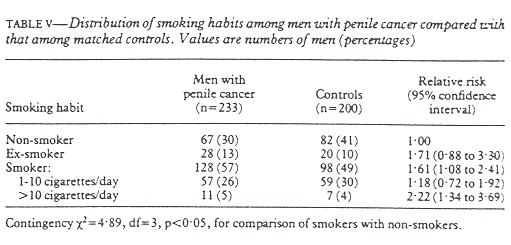
We identified three risk factors, phimosis, balanitis, and smoking, which despite having been statistically controlled for significant confounding events, remained significantly associated with penile cancer. Our initial analyses also showed an association between phimosis and balanitis and phimosis and smoking, but not balanitis and smoking. We then did a logistic regression analysis, adjusting simultaneously for smoking, balanitis, and phimosis to give the genuine risk for each of those factors. For light smokers the risk was 0.98 (95% confidence level 0.68 to 1.42); for smokers smoking 11 or more cigarettes a day 1.53 (1.00 to 2.35); for men with a history of phimosis 57.4 (14.1 to 233); and for those with a history of balanitis 2.44 (1.03-5.78). Despite the adjustment for possible confounding effects of both phimosis and balanitis smoking remained a risk factor for penile cancer: the relative risk in smokers of more than 10 cigararettes a day was 1.53, but the lower limit of the confidence interval was 1.00.
We interpret our results as supporting the hypothesis that smoking is a risk factor for penile cancer. The association between smoking and penile cancer was direct, related to the dose, and independent of other known risk factors, which suggests that smoking is a genuine causal factor. By comparison with the cervix, where high concentrations of nicotine have been found in cervical mucus,10 tobacco products could become concentrated in smegma, making it carcenogenic, especially in men with phimosis. Castonguay et al showed that in rats nitrosamines specific to tobacco were not only excreted by the kidneys but also concentrated in the preputial glands.24
An alternative interpretation is possible, however, because, we studied only living men: theoretically smoking could have prolonged the lives of men with penile cancer, thus explaining the larger number of smokers in this group. This interpretation, however, seems unlikely: there is no other non-hormone dependent cancer in which smoking is known to prolong life, and smokers are known to die earlier than non-smokers. The prevalence of smoking would be expected to be higher in the group of men who had died of penile cancer, but the same would also apply to a control group of dead men, so no bias would be expected for smoking.
There is no evidence that different forms of penile cancer exist with different aetologies. In this study of living men the average age of birth was 1918 and for all cases in Sweden from 1958 to 1980 was 1904. As penile cancer generally has a good prognosis, with up to 90% of patients surviving five years,25 age may be the main factor that differs between the population studied here and a cumulative population of patients with penile cancer.
This study also confirms earlier risk factors for penile cancer (phimosis and balanitis). Phimosis seems to be a genuine risk factor, which probably occurs through the retention of smegma because smegma is not retained by the group at negative risk--that is, those men who were circumcised during childhood. The increasing incidence of early circumcision and better hygienic standards may account for the fall in the incidence of penile cancer in the United States.26 In tests of carcinogenicity of smegma smegma from horses (which have a high incidence of penile cancer) was found to be carcinogenic in mice,27 whereas human smegma was found to be carcinogenic in mice in one study28 but not in another29; in this latter study, however, only four mice were tested so the results are not conclusive.
We did not confirm the hypothesis that penile cancer is a sexually transmitted disease. This may have been because of bias in recalling sexual history, although we might expect recall to be more detailed among patients than controls because they are more likely to try to remember exactly their sexual experiences. In addition, we instructed both patients and controls to leave questions unanswered if there was any shadow of doubt about their answers. It therefore seems plausible that penile cancer is not sexually transmitted.
If so the theory that human papillomavirus is an aetiological agent may be difficult to defend. In 90% of cases of cervical cancer deoxyribonucleic acid from human papillomavirus, mainly types 16 and 18, has been isolated from cancer cells,30 but it has been isolated from only 35 out of 85 (41%) cases of penile cancer tested.12-16 Rather than causing penile cancer human papillomavirus type 16 seems to cause cancer in situ (Bowen's disease)14,31 or bowenoid papulosis.31 This may explain why cervical cancer is transmitted sexually and penile cancer is not. Of course, another type of human papillomavirus with other modes of transmission than sexual might cause penile cancer; the numbers of types of human papillomavirus isolated is steadily increasing and is now more than 40.30. Another explanation may be that infection with the virus is so prevalent in a normal population that measures of sexual behavior do not affect its transmission. (H zur Hausen, personal communication).
In summary, the results of this study suggest that another cancer, penile cancer may be caused by smoking. This association between penile cancer and smoking can be explained biologically and is independent of phimosis; treatment of phimosis alone cannot be expected to rule out the risk caused by smoking. We could not, however, show that penile cancer is a sexually transmitted disease.
We thank Ann-Sofi Axelsson for excellent secretarial help and the Research Council of Delarna for supporting this study.
(Accepted 15 September 1987)
Department of Obstetrics and Gynecology,
Uppsala University, Falu Hospital, S-791 82 Falun, Sweden
DAN HELLBERG, MD
STAFFAN NILSSON, MD, PHD
National Institute of Radiation Protection, Stockholm
JACK VALENTIN, PHD
Department of Urology, Falu Hospital
TORE EKLUND, MD
Correspondence to Dr. Hellberg.
The Circumcision Information and Resource Pages are a not-for-profit educational resource and library. IntactiWiki hosts this website but is not responsible for the content of this site. CIRP makes documents available without charge, for informational purposes only. The contents of this site are not intended to replace the professional medical or legal advice of a licensed practitioner.
© CIRP.org 1996-2025 | Filetree | Please visit our sponsor and host:
IntactiWiki.